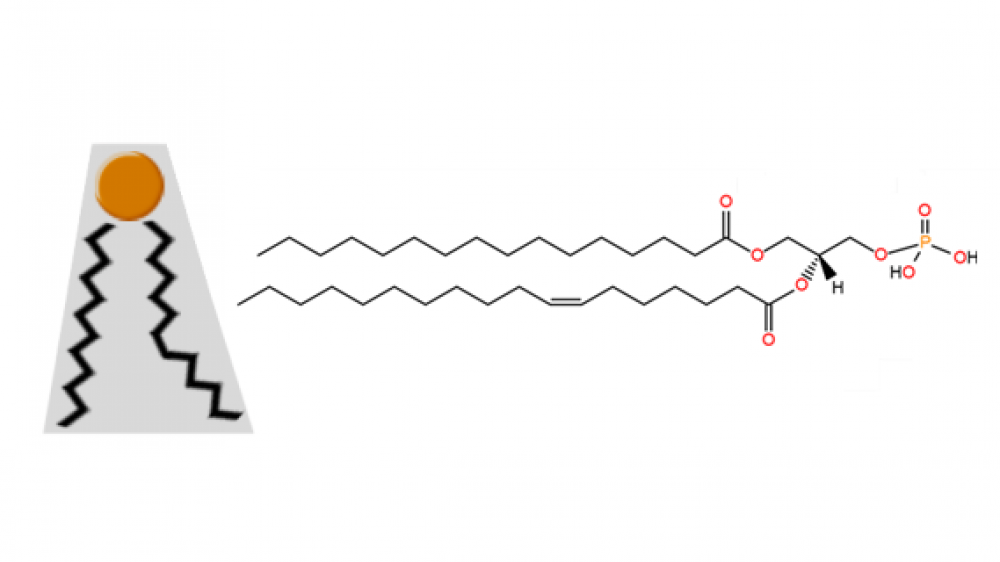
The structure of phosphatidic acid is very simple; indeed, it is the simplest diacyl glycerophospholipid. It consists of a glycerol backbone linked to one phosphate group and two acyl chains. This lipid accounts for approximately 1-4% of the total lipids present in the cells of any living organism.
An example of a molecule of this phospholipid is shown in Figure 1 below. In this case, it contains the acyl chains palmitoyl (16:0) and oleoyl (18:1). The most common fatty acids found in naturally occurring phosphatidic acid molecules are palmitic, oleic, stearic (18:0), linoleic (18:2) and arachidonic (20:4) acids.
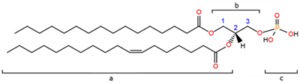
Figure 1. Organic structure of one molecule of phosphatidic acid; a) acyl chains; b) glycerol backbone; c) phosphomonoester group; the carbon molecules of glycerol are numbered in blue.
The small size of its phosphate head group, compared to the size of its base, confers it a particular cone shape (Figure 2 below) that is very important in lipid bilayers. This shape is key to allow the formation of negative curvatures of lipid bilayers that is critical to the fusion and fission of portions of membranes or entire organelles (e.g. plasma membrane or Golgi apparatus), as well as to various biochemical processes happening near cell membranes (e.g. recruitment of signaling proteins).
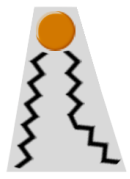
Figure 2. Cone shape of a molecule of phosphatidic acid; the small head group represented in orange; the black zigzag lines represent acyl chains.
The free phosphate group provides phosphatidic acid with a negatively charged head group. These anionic lipids are known to recruit and dock positively charged molecules. Plus, its phosphomonoester group can carry one or two negative charges; inside cells, the physiological pH of different intracellular situations provides the conditions to make phosphatidic acid carry one or two negative charges, which consists on a variable that can be used by certain cell environments to start or end PA-dependent signaling cascades.